The American company “Neuralink” has just conducted the first human transplant of its brain-machine interface (BMI) device, sparking widespread attention. What is the technology behind brain-machine interfaces, and what benefits can they bring to humans? How is the development progressing, and what are the prospects?
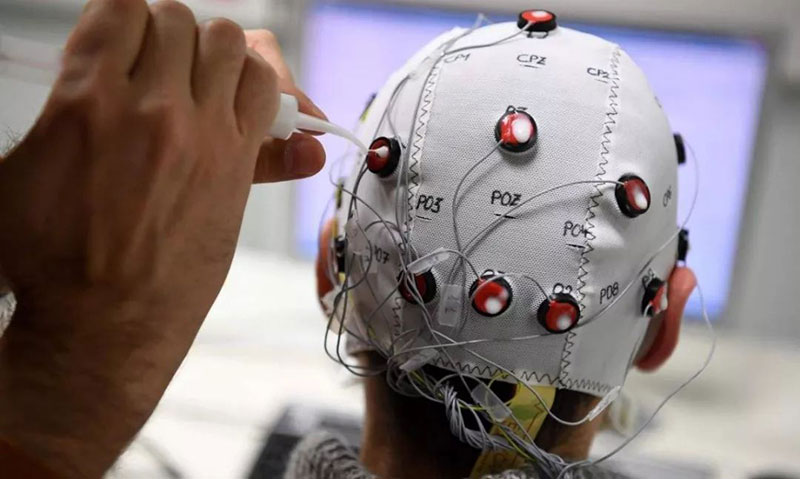
Elon Musk, the renowned American entrepreneur, announced on the 29th that his brain-machine interface company, Neuralink, performed the first human transplant of its BMI device on the 28th, and the recipient is currently recovering well.
According to Musk, Neuralink’s first BMI product is called “MindLink.” After implantation into the brain, this device allows users to control phones and computers through thoughts, enabling control over almost all devices. The initial users of this product are expected to be individuals with limb disabilities.
Brain-machine interface (BMI) is a revolutionary human-machine interaction technology that works by collecting neural signals from the brain and analyzing them to convert into specific commands. Typically, the brain and muscles rely on nerves to output commands to the external environment, but BMI creates a direct connection between the brain and external devices, facilitating direct information exchange between the “brain” and the “machine.”
The crucial functions of BMI devices include aiding in the treatment of memory decline, spinal cord injuries, and other neurological disorders. They help patients with motor function impairments and paralysis regain partial abilities, even assisting them in walking again, ultimately improving and enhancing their quality of life. As BMI technology develops, its potential applications in both medical and non-medical fields continue to expand, including monitoring and assessing brain states, regulating nerves, enhancing sensory capabilities, improving gaming controls, and applications in education and the military.
Currently, BMI technology is categorized into non-invasive, invasive, and semi-invasive types based on the extent of intrusion into the brain. Neuralink’s products fall under the invasive category. Musk stated that preliminary results indicate a promising future for the implanted BMI devices in detecting neuron-related activities.
While BMI devices offer powerful potential functions, concerns and controversies about their safety when implanted in the human body persist. Invasive electrode implantation poses significant risks, such as the potential for immune reactions and the formation of scar tissue, which may affect the quality of electrode signals.
To address these concerns, Neuralink conducted animal experiments. In August 2020, Musk live-streamed a demonstration featuring a pig with a brain implanted BMI device, showcasing real-time reading of brain activity signals. The pig, after the brain implant, exhibited good health with no apparent differences from ordinary pigs. In 2021, there were reports that Neuralink implanted a microchip into a monkey’s brain, enabling it to play video games through thoughts.

Since 2019, Musk has repeatedly predicted that Neuralink would soon receive approval to initiate human clinical trials. However, it wasn’t until May 2023 that the U.S. Food and Drug Administration granted approval for human trials. In September of the same year, the company began recruiting participants for its first BMI clinical trial.
Neuralink describes its research project, formally named “Precision Implantation of Brain-Machine Interfaces,” as an experiment involving fully implantable wireless BMI medical devices. The trial aims to evaluate the safety of implants and surgical robots, assess the initial functionality of BMI, and assist paralyzed individuals in controlling external devices through brain thoughts. In this study, a surgical robot is responsible for implanting ultra-fine flexible threads into brain areas controlling motor intentions, recording brain signals, and wirelessly transmitting them to applications that decode motor intentions.
Neuralink states that this research aims to assist populations with special needs, including those paralyzed due to spinal cord injuries or amyotrophic lateral sclerosis (commonly known as ALS).
Although BMI technology has made significant progress and holds vast potential, overcoming numerous obstacles is necessary before achieving widespread commercial use. These obstacles include not only practical technological bottlenecks but also ethical, privacy, and social fairness issues.
According to U.S. media reports, in addition to Neuralink, several other American companies are developing BMI technology, and some have already conducted human clinical trials.











Leave a comment