Recently, a team led by Shao Yue from the School of Aeronautics and Astronautics at Tsinghua University, in collaboration with a team led by Bai Bing from Kunming University of Science and Technology, Liu Xin from Beijing BGI Life Sciences Institute, and Lin Feng from the Chinese Academy of Sciences (Wenzhou Institute of Science and Technology), have made significant progress in studying the early developmental mechanisms and in vitro reconstruction of the human gastric organ. Using human pluripotent stem cells, they have for the first time established an in vitro model of gastric organ development with a bipolar distribution of the fundus and antrum. This research addresses the paradox of the WNT signaling gradient and establishes a microscale tissue-directed assembly engineering technique, enabling independent gene editing of tissue modules of different lineages within a gastric-like pouch. The research results were recently published in the international journal Nature.
The human stomach is a delicate digestive organ with two regions, the fundus and antrum, arranged in an orderly fashion along the anterior-posterior axis, responsible for secretory and digestive functions, respectively. This asymmetric tissue pattern begins to form as early as the fifth week of embryonic development (fifth week after conception). However, for the past 20 years, a paradox has persisted in the scientific community. Classical developmental biology holds that WNT signaling is an increasing gradient along the anterior-posterior axis, thereby regulating the anterior-posterior patterning of various organs. However, the asymmetric development of the stomach requires a decreasing WNT signaling gradient along the anterior-posterior axis. This “signaling gradient paradox” poses a significant challenge to conventional theories of organ development.
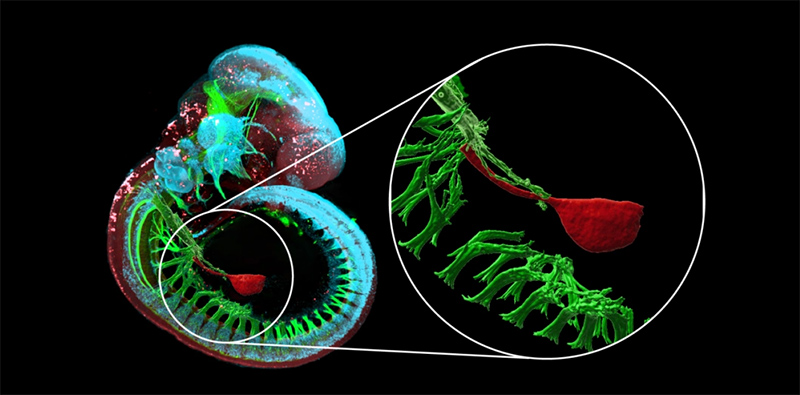
To address this challenge, the research team, drawing on this seemingly contradictory signaling gradient paradox, proposed a novel approach: a novel WNT signaling center may underlie the development of the stomach’s anterior-posterior asymmetric patterning.
To explore this hypothesis, the research team integrated cutting-edge methods and multidisciplinary approaches from mechanics, engineering, and biology. Using biomimetic inspiration, they constructed a three-dimensional environment that simulates in vivo organ development. Using a “multi-lineage coordinated development” strategy, they used human pluripotent stem cells to create, for the first time, a gastric organoid model with a bipolar distribution of the fundus and antrum in vitro.
This model, named the gastric pouch-like model, recreates the asymmetric tissue patterning of early gastric organoid development along the anterior-posterior axis and exhibits high similarity to human and mouse gastric developmental features in molecular, cellular, histological, and anatomical dimensions.
“This study reveals for the first time that neural tissue is an essential signaling center for regulating the anterior-posterior tissue patterning of the gastric organoid. Through its asymmetric geometric relationship with the gastric epithelium during coordinated development, neural tissue induces a WNT signaling gradient that decreases along the anterior-posterior axis, making it a key factor in the formation of the fundus-antrum spatial patterning.” The research team explained that this latest discovery not only resolves the WNT signaling gradient paradox but also provides new principles and methods for constructing high-fidelity gastric organoid models in vitro.












